Abstract
Introduction The fms-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) is the most common recurrent mutation in acute myeloid leukemia (AML). FLT3-ITDs are predominately located in exons 14 and 15 and affect the juxtamembrane domain (JM) of the membranous tyrosine kinase receptor. Oligoclonal FLT3-ITD clone could be detected in single patient. In patients with FLT3-ITD-positive AML, the FLT3-ITD clone could disappear in relapsed AML, the whereas some cases acquired FLT3-ITD in relapsed sample despite the fact that FLT3-ITD was absent in initial AML samples. Clinically, FLT3-ITD was detected by agarose gel or capillary electrophoresis of the polymerase chain reaction (PCR) product. The detection sensitivity of these methods is about 1-5% variant allele frequency (VAF). PCR amplicon sequence using next-generation sequencing (NGS) enabled us to detect dynamics of subclinical minute FLT3-ITD clones. We hypothesized that subclinical minute FLT3-ITD could be detected in clinically FLT3-ITD-negative AML and that the clone might contribute to the emergence of FLT3-ITD on relapse.
Patients and Methods Hokkaido Leukemia Net (HLN) is prospective cohort study collecting acute leukemia samples from affiliated hospitals covering Hokkaido. In this study, we focused on cytogenetically normal (CN) AML without FLT3-ITD diagnosed by electrophoresis. Initial diagnostic samples and relapsed samples were analyzed by the NGS assay to determine whether they had minute FLT3-ITD clones. FLT3 exons 14 and 15 were PCR-amplified and deep-sequenced by Miseq (>20,000 reads). Using an FLT3-ITD-positive AML cell line (MOLM14), the limit of quantitative detection of minute FLT3-ITD was 0.0067%. We defined "minute FLT3-ITD” as one that was not detected by gel electrophoresis but was detected by NGS with VAF of more than 0.0067%. This study was conducted in accordance with the Helsinki Declaration and was approved by the institutional review boards.
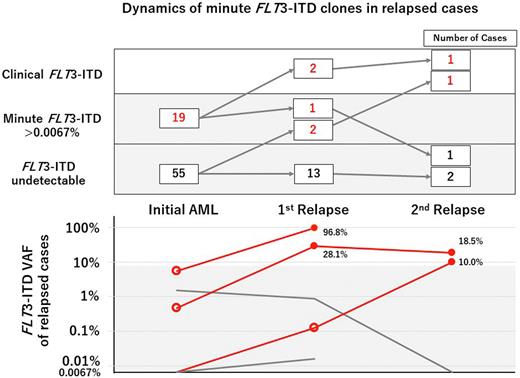
Results A total of 74 FLT3-ITD-negative cases were identified in 112 CN-AML cases registered in HLN from 2017 to 2019 (age range 21-93 years (median 68); 36 males and 38 females). Initial diagnostic samples were analyzed for FLT3-ITD by NGS and 28 independent minute FLT3-ITDs (VAF range: 0.0077-5.4%, median VAF:0.16%) were detected in 19 cases (25.7%). ITD lengths were 18-93 bp (median length: 44 bp), and all minute FLT3-ITDs were in-frame mutations. In 5 of the 19 cases, more than two independent minute FLT3-ITDs were detected. The number of independent ITDs ranged from 1-5 (median number:1) per patient. We compared characteristics of the patients with and those without minute FLT3-ITD. The proportion of BM specimens (89.5% vs 87.3%, P=1.0), white blood cells [23,100 (460-250,900) vs 10,700 (800-174,000), P=0.138], NPM1 mutation positivity (52.6% vs 29.1%, P=0.094), percentage of patients with primary induction failure (47.4% vs 41.8%, P=0.44), and percentage of patients who received allogenic stem cell transplantation (21.1% vs 27.3%, P=0.76) were not significantly different between the minute FLT3-ITD-positive group and the minute FLT3-ITD-negative group. On the other hand, blast percentage (83.0% vs 66.0%, P=0.033) and the rate of relapse within 1 year (36.8% vs 10.9%, P=0.031) were significantly higher in minute the FLT3-ITD-positive group, whereas overall survival rate were not different in the two groups (P=0.78). In a median period of 25 months after initial diagnosis, 22 patients relapsed [7 (36.8%) of 19 minute FLT3-ITD-positive patients and 15 (27.3%) of 55 minutes FLT3-ITD-negative patients]. Of those 22 cases, we collected 23 relapse specimens of 18 cases [1st relapse (rel1), 18; 2nd relapse, (rel2), 5]. FLT3-ITD became clinically positive in 2 cases in rel1 and in 2 cases in rel2 (Figure, red lines). Regarding rel1, 2 of 3 cases in the initial minute FLT3-ITD-positive group developed clinically relevant FLT3-ITD, while none of the 15 cases in the minute FLT3-ITD-negative group developed clinically relevant FLT3-ITD (P=0.020). In both rel1 and rel2, cases that developed clinically positive FLT3-ITD (filled circle) were positive for minute FLT3-ITD at the preceding time point (empty circle).
Conclusion This is the first study clarifying the clonal dynamics of minute FLT3-ITD in clinically FLT3-ITD-negative AML. The clinical significance of an FLT3 inhibitor for patients with minute FLT3-ITD needs to be clarified in the future.
Disclosures
Kondo:Chugai Pharmaceutical: Honoraria; PharmaEssentia Japan: Honoraria; Alexion Pharma: Honoraria. Teshima:Pfizer: Honoraria; Bristol-Myers Squibb: Honoraria; Chugai: Research Funding; Astellas: Research Funding; TEIJIN PHARMA: Research Funding; Fuji Pharma: Research Funding; NIPPON SHINYAKU: Research Funding; Janssen: Other: Manuscript preparation; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Manuscript preparation, Research Funding; Luca Science Inc.: Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck Sharp & Dohme: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Research Funding; Kyowa Kirin: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.